senseBP
The New Standard in Blood Pressure Monitoring is Here
A handheld, cuffless, calibration-free solution built for medical precision and commercial scalability across global health markets.
High blood pressure is the leading cause of premature death worldwide. Yet nearly half of patients do not know they have it, and when they do, accurate monitoring is impractical, uncomfortable, and unreliable.
Why Existing Methods Fall Short
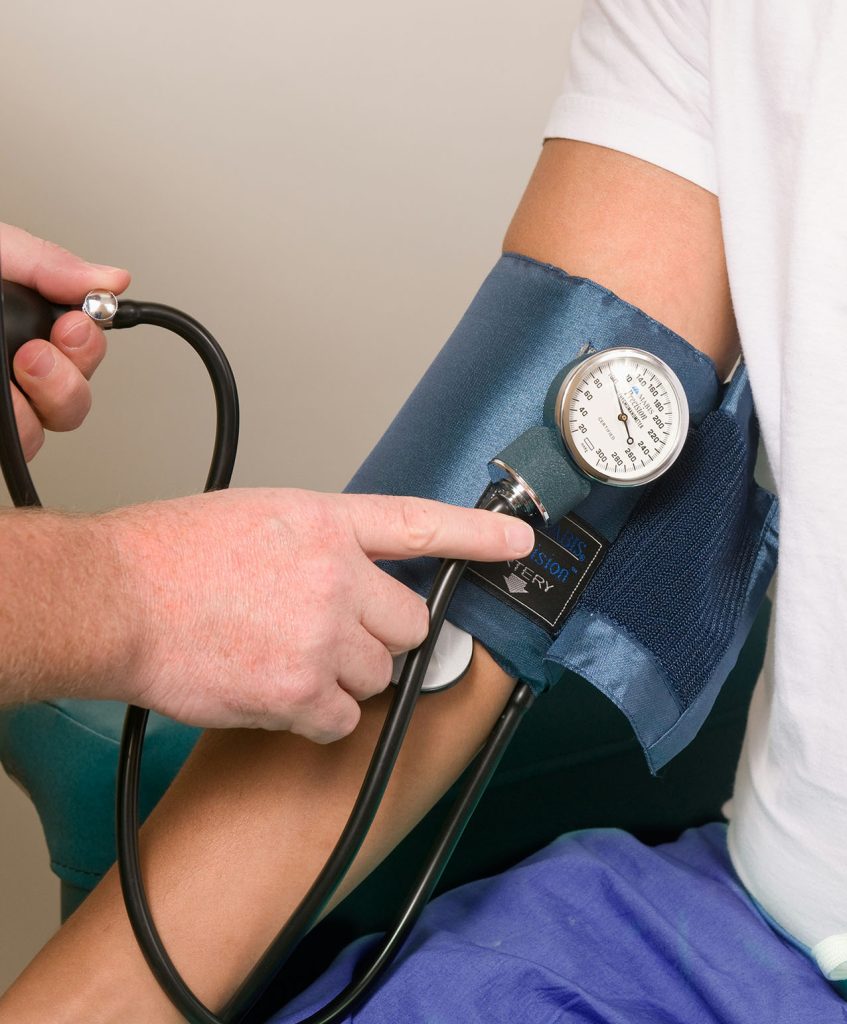
Arterial Lines are impractical. Cuffs Are Cumbersome. Optical Sensors Are Unreliable.
Arterial Lines
Traditional Cuffs
Optical Sensor
Comparison of Blood Pressure Monitoring Methods
Novosound’s ultrasound sensors deliver what others cannot: accuracy, usability, and comfort without compromise, all in one device.
Cuff | Optical* | Arterial Line | senseBP Ultrasound | |
|---|---|---|---|---|
Comfortable** | ||||
Reliable | ||||
Practical | ||||
Calibration-Free |
*Optical refers to PPG (photoplethysmography) wearables, which estimate BP indirectly from light-based pulse signals; performance can vary with motion, skin perfusion/temperature, and skin tone. Most solutions require initial or periodic cuff calibration rather than being calibration-free.
**Comfortable refers to patient comfort/tolerance during measurement — whether a single reading or repeated monitoring. Upper-arm cuffs can be painful or poorly tolerated in some groups (frail/older adults, palliative patients, those with neuropathy/lymphoedema, or post-surgery), causing sleep disturbance, skin marking/bruising, and agitation. Wearable ultrasound avoids arm compression.
Our Solution
Meet the Handheld Device Designed for Daily Use
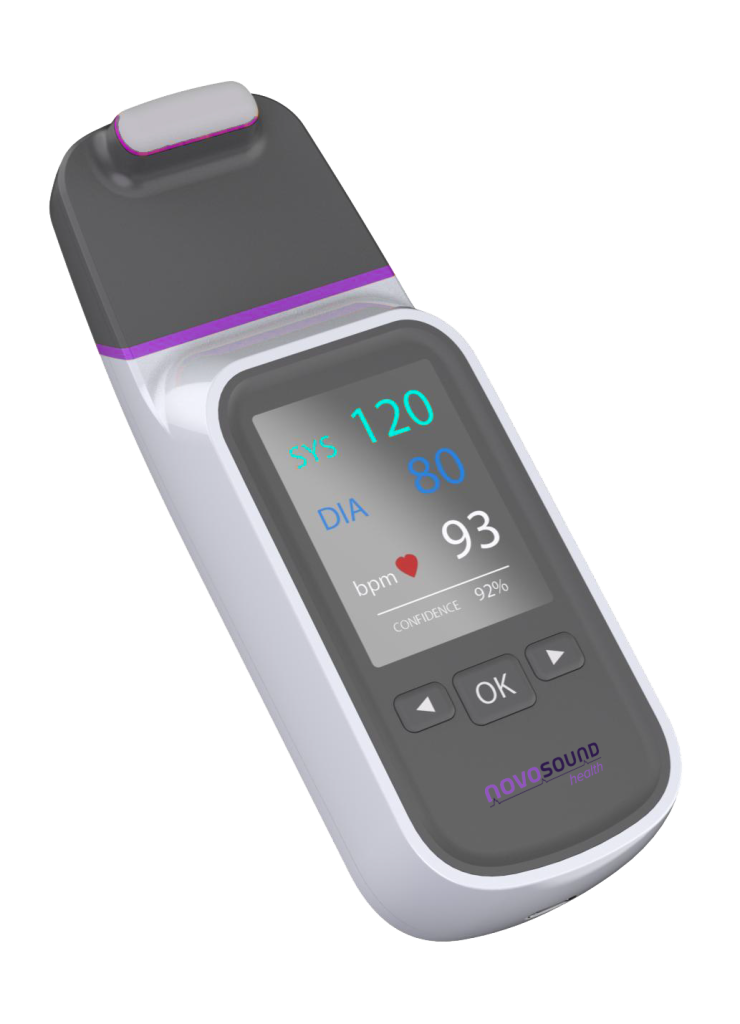
senseBP
Imagine an at-risk patient checking their blood pressure daily from hospital or home, no cuffs, no wires, no calibration. senseBP makes it possible.
- Cuffless and calibration-free
- Accurate to ±5 mmHg
- Lightweight and portable
- Quick and simple to use

How It Works
Powered by Patented Ultrasound Technology it provides a high-performance ultrasound solution.
Novosound’s flexible ultrasound array captures direct measurements of arterial movement enabling real-time, patient-friendly blood pressure monitoring with high precision.
Why Now? The shift is already happening
- Global demand is rising for blood pressure monitoring that is accurate, convenient, and accessible.
- Healthcare is moving rapidly toward personalized, sensor-driven technologies that deliver real-time insights. The market for health monitoring devices is expanding fast, driven by rising chronic conditions, digital health adoption, and consumer demand for proactive care.
- Blood pressure remains a key metric, essential, yet underserved by current tools.
- Novosound is uniquely positioned at the intersection of medical innovation and commercial pragmatism. Our proprietary ultrasound technology begins with blood pressure but opens the door to scalable, sensor-based health solutions across the continuum of care.
The Device Designed for Daily Use

Cuffless & calibration free

As accurate as a cuff
Quick & simple to use
Lightweight & portable
Novosound Health Leadership Team
Gary Beale
John Cameron
Mairi MacFadyen
Dan Irving
Dr Praveen Ashok
Dr Manuel Pelayo
This is our animation
You should give it a watch
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
